Description
This research area addresses the theoretical and experimental understanding of motor and balance dysfunction and the effect of disorders on patients in their daily activities.
We develop a wide range of multi-modal assistive tools to support people with neurological movement disorders and psychological disorders. We address the preclinical and clinical phases of various neurological disorders, including Cerebellar Ataxia, Hereditary Spastic Paraplegia, Parkinson’s disease, and Apraxia. Furthermore, multi-modal systems are used to improve therapeutic interventions for mentally ill subjects, e.g., obsessive-compulsive disorders.
Researchers
Current Projects
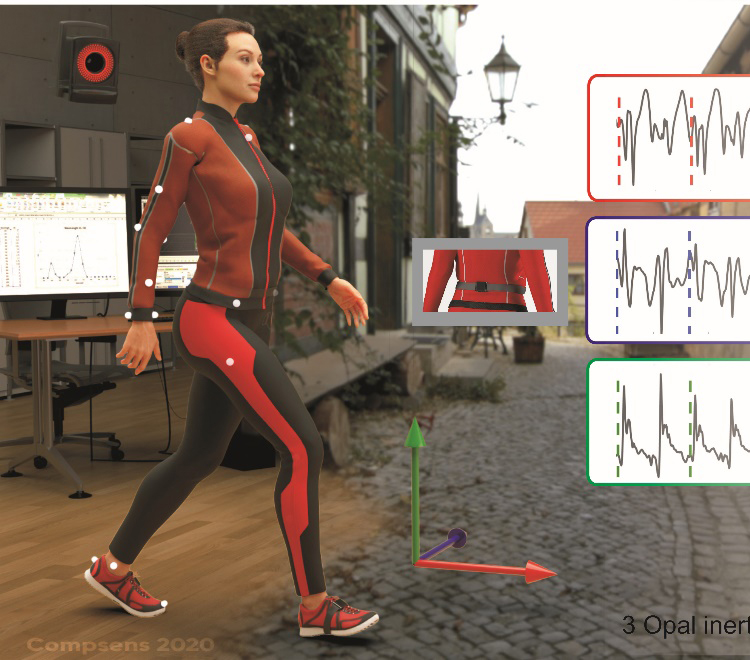
Real-life gait assessment in degenerative cerebellar ataxia: Towards ecologically valid biomarkers
In order to establish ecologically valid biomarkers evaluating treatment-responses really in the patients’ everyday life, we develop multi-variate measures of ataxic gait using wearable sensors, which demonstrate high sensitivity to small differences in disease severity in real-life walking.
Read more
SSTeP KiZ: smart sensor technology in tele-psychotherapy for children and adolescents with obsessive-compulsive disorder
With sensors that can be worn in everyday life and an intelligent analysis of multi-modal sensor data, SSTeP KiZ aims to significantly improve the treatment options for patients with obsessive-compulsive disorder. We support telemedical treatment of affected children and adolescents in their home environment by integrating data collected with wearables.
Read more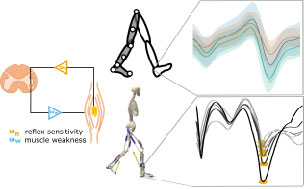
Gait in hereditary spastic paraplegia – from axonal degeneration to movement disorder
In Hereditary Spastic Paraplegia (HSP) type 4 (SPG4 / SPAST) a length-dependent axonal degeneration in the cortico-spinal tract leads to progressing symptoms of hyperreflexia, muscle weakness, and spasticity of lower extremities. The therapeutical potential for future intervention is likely most promising in the early stages of HSP. Therefore, it is crucial to identify and quantify first changes already in the prodromal phase of HSP patients.
Read more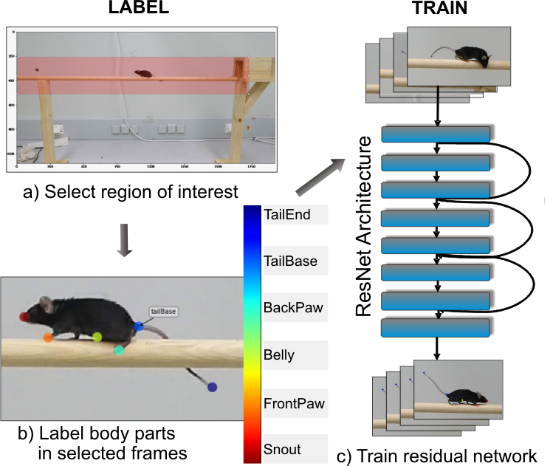
Detecting and Quantifying Ataxia-Related Motor Impairments in Rodents Using Markerless Motion Tracking With Deep Neural Networks
Animal models of adult-onset neurodegenerative diseases have significantly enhanced the understanding of the molecular (patho-)mechanisms and have offered enormous potential for therapeutic target evaluation in many neurodegenerative diseases.
Read moreFinished Projects
Cerebellar involvement in the facilitation of action perception by concurrent motor activity
The execution of motor behavior influences concurrent visual action observation, and especially the perception of biological motion. Exploiting Virtual Reality technology, we have studied how the cerebellum contributes to action-perception coupling, comparing cerebellar patients with controls.
Read more
Smart sensor technology in telepsychotherapy for children and adolescents SStep-KiZ
Through the use of sensors that can be worn in everyday life and an intelligent analysis of multi-modal sensor data, SSTeP-KiZ aims to significantly improve the treatment options for mentally ill children and adolescents with obsessive-compulsive disorders.
Read more
Development of exergames for the motor training in cerebellar ataxia
Computer games provide a possibility to enhance physiotherapeutic training and to increase the motivation of patients in such training. We develop own games that are optimally adapted to the needs of different patient groups, and specifically cerebellar ataxia patients.
Read more
The influence of focal cerebellar lesions on the coordination in walking
In this study we examined patients with focal cerebellar lesions in order to investigate the influence of different regions of the cerebellum on the performance in a working memory task (n-back task), as well as on gait variability and gait stability during dual task walking.
Read more
Cost-efficient system for movement quantification in neurology
The Microsoft Kinect sensor for computer games allows robust body motion tracking for relatively low cost. We use this technology for the analysis of patient movements and develop cheap systems for the quantification of movement deficits that can be deployed at home or simultaneously at multiple places for multi-center studies.
Read moreDesign and development of a mobile robot supporting the rehabilitation of free walking
Current walking rehabilitation is mainly restricted to treadmill walking in order to train basic rhythmic walking patterns. Together with the Fraunhofer IPA (Stuttgart) we designed a new robot platform to train walking in complex situations (turning, standing up, etc.).
Read more
Influence of action execution on biological motion perception
The perception and execution of motor actions are tightly interlinked, and numerous experiments suggest the existence of common sensory-motor representations.Using a virtual-reality setup we aim to investigate the influence of self-generated body motion on the perception of online generated biological motion in combined motor behaviour and psychophysical studies.
Read more
Motor learning and the functional role of the cerebellum
The cerebellum plays an essential role in motor learning. Combining psychophysics and neuropsychological studies in patients we investigate different types of motor learning mechanisms and the role of the cerebellum.
Read more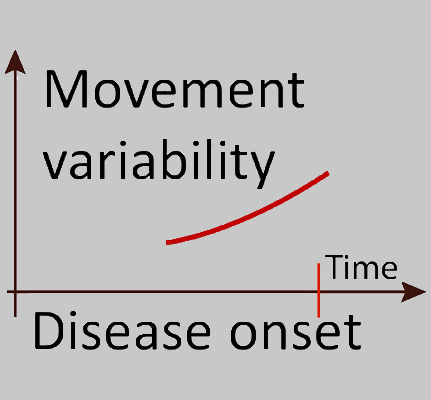
Quantification of subtle motor changes in preclinical stages of neurodegenerative diseases
Movement disorders such as cerebellar ataxia or Parkinon’s disease result in subtle degradations of motor behavior already long time before the become clinically manifest. Using motion capture technology and machine learning, we try to identify such subtle preclinical motor symptoms.
Read more
Rehabilitation training exploiting physiotherapy, computer games and biofeedback
The symptoms o movement disorders, such as cerebellar ataxia or Parkinon’s disease, can be partially improved y motor training. We have shown that (opposed to the classical view) physiotherapy results in substantial and enduring benefits for patients with cerebellar ataxia, if such training is continuously administered. We exploit biofeedback and computer games to improve such training.
Read morePublications
Should Patients Suffering from Degenerative Cerebellar Ataxia Switch from Balance Training to Aerobic Exercise?
Digital Outcomes of Upper Limb Ataxia Capture Meaningful Longitudinal Change and Treatment Response
Abstract Background Digital-motor outcomes promise better responsiveness than clinician-reported outcomes in ataxia trials. However, their patient meaningfulness and sensitivity to change remain to be demonstrated, particularly in the upper limb domain. Objectives Validation of quantitative motor (Q-Motor) assessment for upper limb ataxia against patient-reported outcomes and regarding sensitivity to both longitudinal and treatment-induced change, the latter in n-of-1 treatment settings. Methods Single-center longitudinal assessment of finger tapping, diadochokinesia, grip-lift, spiral drawing, and target reaching in (1) 36 cross-genotype ataxia patients and 20 controls, validating digital measures for correlations with patient-reported outcome measure (PROM)-ataxia, 2-weeks test–retest reliability, and sensitivity to change within a trial-relevant 1-year follow-up, anchored in Patient Global Impression of Change (PGI-C); and (2) two patients with spinocerebellar ataxia type 27B (SCA27B) on versus off treatment with 4-aminopyridine. Results Twenty-four digital measures correlated with the PROM-ataxia upper-limb composite (|ρ| = 0.4–0.7) and had excellent test–retest reliability (ICC = 0.91–0.99). Correlations to individual PROM-ataxia items were specific for functional impairment the respective measure was hypothesized to capture. Speed of finger tapping and diadochokinesia, and smoothness of target reaching (spectral arc length of movement in three dimensions [SPARC3D]) captured 1-year progression in ataxia patients (|rprb| = 0.38–0.51), and specifically in patients with worsening PGI-C. Estimated sample sizes to detect longitudinal change were lower for digital than clinical outcomes (SPARC3D: n = 33, Scale for the Assessment and Rating of Ataxia (SARA): n = 79, nine-hole peg-test: n = 214). Speed of diadochokinesia, stability of grip-lift, and variability of target reaching captured treatment responses to 4-aminopyridine in SCA27B, exceeding minimal detectable and minimal important change. Conclusion Digital upper limb measures capture patient-meaningful 1-year longitudinal and treatment-induced change, and are therefore promising outcomes for upcoming ataxia trials. © 2025 The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Turns increase the impact of impaired eye movements on locomotion in cerebellar ataxia
BACKGROUND AND AIM: Turning movements are a highly relevant component of everyday walking behavior, since 35-45\% of steps are taken during turning. Turning movements are thought to be more challenging in terms of dynamic balance than straight walking, as they require more anticipatory postural adjustments and trunk-limb coordination strategies. In addition, certain types of degenerative cerebellar ataxias are associated with disturbances in eye movements such as nystagmus and disturbed VOR reflexes, which occur particularly during head rotation and peripheral gaze and may therefore affect turning more than straight walking. In this study, we compared the turning movements of SCA27B ataxia patients with downbeat nystagmus (DBN) to those of patients with spinocerebellar ataxia (SCA, types 1, 2, 3, 6) without nystagmus and investigated the influence of the drug 4-aminopyridine (4AP) on the reduction of DBN during turning movements. METHODS: We performed a cross-sectional analysis of motion data collected by three body-worn inertial sensors from subjects with SCA1, 2, 3, 6 (n = 359, SARA = 6.81) as well as SCA27B (n = 49, SARA = 7.0) in two conditions: a) lab-based supervised walking of a 60m corridor at preferred speed, b) lab-based turn task, i.e., subjects were instructed to walk along a T-junction of a corridor, including several 90° turns. Turning analysis included standard measures (i.e., mean and peak angular velocity (MAV, PAV), turn duration (TD), number of steps during turning (NoS)) and a measure quantifying dynamic balance during turning (lateral velocity change, LVC), which has been shown to be sensitive to ataxic-specific changes in turning and has strong correlations with self-reported balance confidence as measured by the ABC score. RESULTS: Turn analysis of the LVC revealed significantly greater impairments during lab-based 90° turning (p = 0.001, Cliff’s δ = 0.45) in SCA27B patients with DBN (n = 18) than in SCA1/2/3/6 patients without oculomotor impairment (n = 359). Small or no effects were found for the standard turn parameters (e.g., PAV (p = 0.49, δ = 0.10), TD (p = 0.30, δ = -0.15). Single-subject analysis of a 4AP-treated SCA27B patient with prominent DBN at right and left gaze directions showed both a reduction in DBN and LVC in the ON treatment phase compared to pre-treatment. The slow phase velocity was reduced by 16.1\% in right and by 51.2\% in left gaze. Accordingly, the LVC decreased by -0.46 m/s (-85.3\%) during right and by -0.51 m/s (-98.38\%) during left turns. Here, no improvements were found for the standard turn parameters. CONCLUSIONS: Ataxia-related oculomotor impairments may increase abnormalities in dynamic balance control during turning, which are not reflected in common compensatory strategies such as slowing down and taking smaller steps. The 4AP-induced reduction in DBN in SCA27B patients improves turning performance, with potentially beneficial implications for everyday walking behavior.
Understanding the relationship of static and dynamic balance measures in ataxic stance and gait at different disease stages
BACKGROUND AND AIM: Ataxic gait is typically characterized by an unstable, stumbling gait, increased step width, and high gait variability. The characteristic high variability is thought to result from the complex interaction between cerebellar-induced deficits in balance control and multi-joint coordination, the compensatory strategies used, and inaccurate postural adjustments to the apparent loss of balance. The interplay and relative importance of these individual factors and their development over the course of the disease are not fully understood. Clarifying their relationship during disease progression would allow both efficient neurorehabilitation and the development of disease-phase sensitive performance markers for clinical trials. Here, we aimed to investigate the role of ataxia-specific balance dysfunction in static (stance) and dynamic (gait) conditions, particularly in very early and pre-symptomatic disease stages (i.e., mutation carriers without clinical manifestation). METHODS: We assessed static and dynamic balance of subjects with degenerative cerebellar ataxia at baseline and 1-year follow-up using three body-worn inertial sensors. Stance conditions included natural stance and feet together stance with eyes opened and eyes closed. As a measure of static balance performance we used the sway path length (SPL) based on the hip sensor. Walking was performed in laboratory settings, i.e., supervised straight walking of a 60m corridor at preferred speed, and unsupervised in real life. The compound measure of spatial step variability (SPcmp), which integrates step length variability and lateral step deviation, served as a measure of ataxia-specific gait variability. RESULTS: Cross-sectional analysis of symptomatic ataxia patients (n = 44, SARA = 10.1) revealed correlations between SPL during natural stance and SPcmp during walking, with increasing effects moving from laboratory (r = 0.36, p {\textless} 0.0001) to real-life conditions (r = 0.51, p {\textless} 0.0001). For the group of pre-ataxic mutations carriers (n = 33, SARA = 0.7) we saw a strong trend for the relation of gait variability and sway in a stance task with increased complexity (i.e., feet together, eyes closed) (r = 0.25, p = 0.06). The relation was particularly evident longitudinally when 1-year changes in stance sway and gait variability were correlated (r = 0.44, p = 0.01). CONCLUSIONS: We were able to identify specific influences of the static balance mechanism on gait in pre-symptomatic mutation carriers, suggesting that alterations in balance control mechanisms already play a verifiable role in pre-symptomatic and very early disease stages, whereas cerebellar-induced deficits in balance control and multi-joint coordination and compensatory strategies such as slowing down may have a greater influence in later disease stages. This highlights the importance of static stance testing and related balance exercises in rehabilitation, particularly in pre-symptomatic and early disease stages.
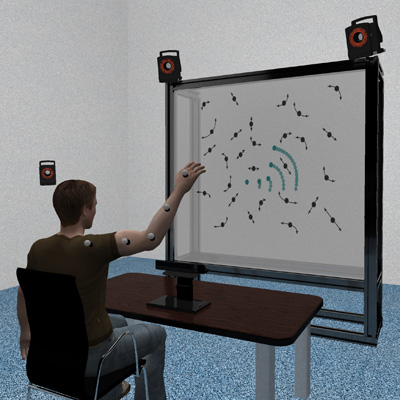
iAssistADL: Intelligent assistive device for patients with neuro- degenerative movement disorder: Concepts and first implementations
Upper-limb activities of daily living like eating and drinking are crucial for self-determination and autonomy and, thus, quality of life. Patients with neurodegenerative diseases such as Parkinson’s disease, multiple sclerosis or cerebellar ataxia are often severely impaired in performing these activities of daily living. While these patients are still able to plan motor actions, and their muscle strength is rarely impaired, tremor or overshooting movements disturb the intended movements. This occurs progressively in the course of disease in a way that inde- pendent eating and drinking becomes increasingly difficult. The goal of this research project is to develop a non-invasive assistive device suppressing pathological movement components while allowing intended movement. The newly designed hardware will be controlled by a combination of computational methods to detect user intention, detect pathological movement components within intended movements, and predict the required correction forces for several upper-limb activities of daily living. In this manuscript, we will describe concepts of control hard- and software as well as first implementation and experiments with the individual components we plan to integrate in the future.
